Executive Summary
This report highlights the successful development and large-scale production of high-quality antimicrobial zinc oxide nanoparticles (ZnO NPs), transitioning from small laboratory batches to full industrial manufacturing. Using an automated flow-reactor system, we optimized ZnO to achieve superior antimicrobial properties, making it ideal for use in coatings, medical applications, and advanced materials.
Unlike conventional ZnO, which is typically spherical, our research found that a flake-like structure provides stronger antimicrobial effects by disrupting bacterial membranes more effectively. To ensure consistent quality, we closely monitored reaction conditions, purity, and product performance at all production stages. Advanced testing confirmed that ZnO NPs produced at different scales remained highly effective, stable, and safe.
Beyond scientific improvements, this work also brings major economic benefits. By automating and optimizing the production process, we reduced costs by 86% and shortened the time required for large-scale manufacturing from 5 years to just 6 months. This breakthrough makes it possible to produce high-performance ZnO NPs more efficiently and affordably, supporting their use in industries requiring antimicrobial and UV-protective solutions.
Introduction
Zinc oxide (ZnO) is a well-known material used across various industries due to its UV-blocking, antibacterial, and conductive properties. In skincare and sunscreens, it appears as nano- or microparticles, providing protection from UV rays while being gentle on the skin. Its antimicrobial properties make it ideal for use in medical dressings, antibacterial paints, and food packaging due to its low toxicity to humans. In the rubber and plastic industries, ZnO powder strengthens materials, improves UV and heat resistance, and enhances durability in products such as tires and synthetic leather [1,2].
In electronics, ZnO is used in thin-film form or as doped ZnO to improve electrical properties in touchscreens, solar panels, and sensors. Its ability to store and transfer energy also makes it valuable in zinc-based batteries. Depending on the application, ZnO is used as nanoparticles, powders, or coatings, each offering specific benefits. This adaptability makes zinc oxide an essential component in modern technology, healthcare, and everyday products [3].
Producing high-quality zinc oxide (ZnO) requires careful control over purity, particle size, and production methods. Impurities such as lead and iron can affect performance, making purification essential, especially for applications in cosmetics, electronics, and pharmaceuticals. Additionally, maintaining uniform particle size and morphology is critical for optimizing ZnO’s properties in UV protection, conductivity, and reactivity. Advanced synthesis techniques, such as hydrothermal or flame spray pyrolysis, help achieve better consistency and purity. Unfortunately, these methods are costly and ineffective when ZnO needs to be produced at a large scale [4].
Another challenge is preventing nanoparticle aggregation, which can reduce ZnO’s effectiveness in coatings, sunscreens, and electronic applications. Surface modification and dispersion techniques help improve stability [5]. Cost and scalability also play a role, as producing high-purity or nanostructured ZnO can be expensive. Process optimization and automation are necessary to maintain quality while keeping production economically viable. These factors make high-quality ZnO production both a technical and economic challenge, requiring innovation in materials processing [6].
Herein, we describe our journey in optimizing high-quality ZnO production, transitioning from R&D-scale synthesis to ton-scale mass manufacturing of highly antimicrobial material. This achievement was made possible through the versatile design and advanced automation of flow-reactor systems developed at Accelerated Materials.
Challenges and Problem Statement
Producing high-quality antimicrobial zinc oxide (ZnO) at scale presents multiple challenges, including maintaining purity, optimizing particle size, ensuring stable antimicrobial performance, and meeting regulatory standards. Purity is a critical factor, as even trace amounts of heavy metals like lead or cadmium can compromise safety and regulatory compliance, particularly for medical and cosmetic applications. Achieving a controlled and uniform particle size is also essential, as smaller nanoparticles exhibit higher antimicrobial activity but are prone to aggregation, which reduces effectiveness in formulations such as coatings, paints, and textiles.
Another major challenge is optimizing the nanoparticle morphology, which drives ZnO’s antimicrobial properties. A release that is too rapid can cause cytotoxicity, while a release that is too slow reduces efficacy. Additionally, ZnO must remain stable over time, resisting agglomeration and degradation under various environmental conditions. Ensuring compatibility with different product formulations—whether in dispersions, solid composites, or sprays—also requires tailored surface treatments.
Scaling up production adds further complexity, as traditional batch methods may not provide the consistency needed for industrial applications. Continuous flow-reactor systems offer better control over ZnO’s properties but require significant process optimization. Finally, regulatory hurdles vary by region and application, with agencies such as the EPA, FDA, and EU REACH requiring extensive toxicity, efficacy, and environmental impact studies before market approval. Addressing these challenges requires a combination of advanced synthesis methods, process automation, and strategic regulatory planning to ensure ZnO remains both effective and commercially viable.
We have summarized the target specifications for ZnO that should meet antimicrobial activity, purity, regulatory compliance, and particle size in the following table.
Summary of Key Metrics for High-Quality Antimicrobial ZnO [1]:
| Criterion | Optimal Range/Requirement |
| Purity | ≥ 99% (low heavy metals) |
| Particle Size | < 100 nm |
| Morphology | Controlled (spherical, rod, or plate-like) |
| Antimicrobial Efficacy | Microbial growth reduction > 99.9% (ISO 22196) |
| Formulation Compatibility | Easily mixed with coating formulations |
| Toxicity | Low cytotoxicity, compliant with FDA/REACH |
| Environmental Safety | Eco-friendly synthesis, minimal environmental impact |
| Scalability | Maintaining quality at scale |
Methodology
Our journey from gram- to ton-scale production leveraged our 1 kg/day (K1), 10 kg/day (K10), and 100 kg/day (K100) continuous flow systems. The K1 system served as an R&D tool for initial process development, while the K10 and K100 systems were optimized for pilot-scale sample production and mass manufacturing, respectively. The overall ZnO development and scale-up process is divided into distinct steps, as illustrated in Scheme 1, with each stage incorporating key performance metrics to ensure product quality and consistency. These metrics include purity, morphology, particle size, crystallinity, and biological activity, which are evaluated using advanced analytical techniques to maintain high standards across all production scales.
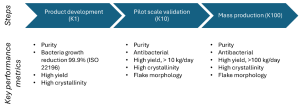
Scheme 1. Steps and key performance criteria for high quality antimicrobial ZnO development and scale up.
Scheme 2 illustrates the stages of the ZnO development and scale-up process, ensuring rigorous quality control at each stage. Reagent concentrations are verified through solution titration, while reaction stability is monitored via continuous pH measurement. The yield is determined by measuring solid content, and product purity is assessed through low conductivity analysis of the dispersion. Finally, the ZnO product undergoes comprehensive analytical characterization, including morphology, particle size, crystallinity, and biological activity evaluation, ensuring consistency and performance across scales.
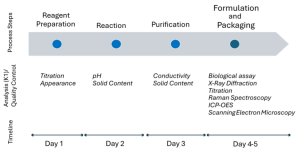
Scheme 2. Process flow for ZnO optimization and scale-up.
Step 1: R&D and Formulation Development
We began each project with R&D studies on the efficacy and viability of various zinc oxide (ZnO) nanoparticle formulations synthesized using our gram-scale K1 system. This led to the development of ZArmour, a high-performance ZnO additive tailored for antimicrobial coatings. Our research identified that high-aspect-ratio, flake-like ZnO—which naturally agglomerates into high-surface-area structures—delivers superior antimicrobial and UV absorption properties. The increased surface charge and localized electric field effects enhance antimicrobial performance by disrupting microbial membranes, making it significantly more effective than conventional spherical ZnO, which is widely used in industry. For more details, you may refer to our publication, “Pushing Nanomaterials up to the Kilogram Scale – An Accelerated Approach for Synthesizing Antimicrobial ZnO with High-Shear Reactors, Machine Learning, and High-Throughput Analysis” [7].
Step 2: Pilot-Scale Production
After validating ZnO formulations at the K1 scale, we translated the optimized process parameters to the K10 system, enabling kilogram-scale production for industry partner evaluations. This step ensured scalability, reproducibility, and consistency in ZnO synthesis before proceeding to larger-scale manufacturing.
Step 3: Mass Production at Ton Scale
With successful pilot-scale production, we systematically scaled up to the K100 system, which integrates multiple K10 reactors in parallel. This modular design allowed us to maintain critical process parameters—including flow rates, temperature control, and reagent concentrations—ensuring that ZnO’s structural and functional properties were preserved during ton-scale manufacturing. This seamless scale-up strategy guarantees product quality, reproducibility, and efficiency while meeting industry demands for high-performance ZnO materials.
Results analysis and quality control:
At every stage, rigorous analytical techniques were implemented to ensure that the ZnO produced met the required specifications and remained consistent across the K1, K10, and K100 scales. Key analytical methods and performance criteria included:
- Reagent Purity – Verified by titration to ensure the correct reagent concentrations.
- Morphology and Particle Size – Assessed using Scanning Electron Microscopy (SEM) to confirm the flake-like particle structure.
- Crystallinity – Evaluated with X-ray Diffraction (XRD) to confirm the zincite crystalline structure of ZnO.
- Qualitative Antimicrobial Activity – Tested using the Disk Diffusion Method on E. coli ATCC 8739, observing inhibition zones. As a positive control Cetyltrimethylammonium Bromide (CTAB) is used.
- Quantitative Antimicrobial Activity – Measured according to ISO 22196 (Assessment of antibacterial activity on plastics and other non-porous surfaces) for paint formulations.
Results and Discussion
Effect of ZnO morphology on antimicrobial properties
Utilizing our K1 system, we have synthesized a variety of ZnO morphologies, as shown in Figure 1. It was observed that by varying reagent concentrations and flow rates, it is possible to produce versatile structures such as spheres, flakes, stars, and snowballs (Figure 1).
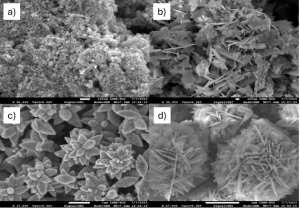
Figure 1. SEM images of various morphologies are synthesized with K1 system: a) Spheres, b) Flakes, c) Stars, d) Snowballs.
The disk diffusion test (also known as the Kirby-Bauer test) is a laboratory method used to evaluate the antimicrobial effectiveness of antibiotics against bacterial strains. In this test, small paper disks impregnated with specific biocides are placed on an agar plate that has been evenly inoculated with the target bacteria. As the bacteria grow, the biocide diffuses into the surrounding medium, creating a zone of inhibition—a clear area around the disk where bacterial growth is suppressed. The size of this zone is measured and compared to standardized charts to determine bacterial susceptibility or resistance to the biocide. An example of the ZnO inhibition zone created against E. coli is shown in Figure 2.
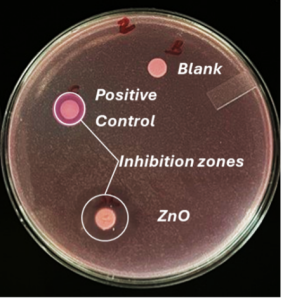
Figure 2. Inhibition zone formation around ZnO-flakes and positive control.
Qualitative disk diffusion tests showed that nano-flakes have the largest inhibition diameter, while stars did not exhibit any antimicrobial activity. The results are shown in Figure 2.
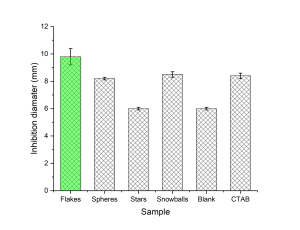
Figure 3. Inhibition diameter for various morphologies of ZnO.
After qualitative assessment, we evaluated how ZnO flakes perform within paint formulation and at what concentration they would be sufficient to achieve 99.9% bacteria growth reduction. Thus, ZnO was mixed with pure polymethylmethacrylate (PMMA) latex dispersion at 0.1%, 0.5%, and 1.2% wt%. Quantitative bacteria growth assessment was done in accordance with ISO 22196, and the results are shown in Figure 3. It is clearly seen that even the addition of 0.1% wt% of ZnO flakes (Figure 3c) slightly reduces bacteria growth, while at the concentration of 1.2% wt%, no growth is observed (Figure 3f).
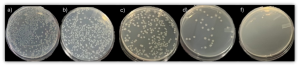
Figure 4. Quantitative bacteria growth assessment was done in accordance with ISO 22196, where a) blank, b) pure PMMA, c) PMMA + ZnO 0.1 wt%, d) PMMA + ZnO 0.5 wt%, e) PMMA + ZnO 1.2 wt%
Pilot and manufacturing scale production
After optimizing process parameters on the gram scale, they can be rapidly translated to pilot and manufacturing systems (K10 and K100) within several months. The quality of large-scale samples was verified with XRD, SEM, and the Disk Diffusion Test and compared with the K1 product.
From the SEM images shown in Figure 4, we can observe that the obtained samples have a similar aggregated flake morphology across K1, K10, and K100 experiments under our process conditions. All samples have primary particles with a flake morphology, which agglomerates into spherical globules when dried. These high-aspect-ratio and high-surface-area structures are key to obtaining high antimicrobial activity and UV absorption.

Figure 5. SEM images for ZnO samples obtained at same process parameters on various K-systems: a) K1, b) K10, c) K100.
X-ray diffraction was the most critical measurement in identifying the formation of pure, crystalline ZnO. ZnO, or “zincite,” has a characteristic structure, with peaks shown in Figure 5. All XRD patterns from the K1, K10, and K100 systems preserve this highly pure structure, which is particularly difficult with ZnO due to the tendency to also form Zn(OH)₂ in batch systems. XRD also shows a uniform crystallite size of about 20 nm across K1, K10, and K100.
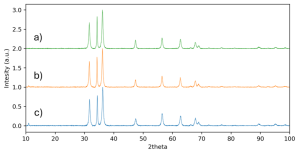
Figure 6. XRD patterns for ZnO synthesized in a) K1, b) K10, c) K100 systems.
Antimicrobial tests across samples from K1, K10, and K100 synthesis give a constant reading of 10 mm for each sample, as seen in Figure 6. Each test has been passed, indicating a high level of antimicrobial activity, comparable to conventional organic antimicrobials, as shown in Table 1.
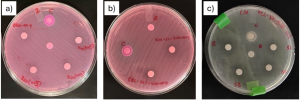
Figure 7. Antimicrobial assay results for ZnO over K1, K10 and K100 trials.
Table 1. Quality control results for antimicrobial activity across 1 kg/day, 10 kg/day and 100 kg/day scales
| Scale | Average Antimicrobial Score | Result |
| K1 | 10 | PASS |
| K10 | 10 | PASS |
| K100 | 10 | PASS |
We also conducted a technoeconomic assessment to show that with this approach, we significantly reduced the time to scale compared to conventional batch reactor methods. As seen in Table 2, we reduced the scale-up investment cost by up to 86%, operating costs by up to 55%, and ultimately reduced the time to breakeven for manufacturers by 94%. In other words, with our system, manufacturers can scale up in 6 months instead of 5 years.
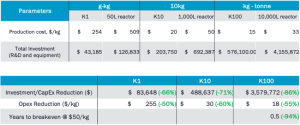
Table 2. Technoeconomic analysis results for the synthesis of nanoparticle ZnO on the K-series platform vs. conventional batch production techniques.
Conclusion
In this study, we successfully optimized and scaled the production of high-performance antimicrobial zinc oxide (ZnO) from laboratory scale (K1, 1 kg/day) to pilot scale (K10, 10 kg/day) and mass manufacturing scale (K100, 100 kg/day). Through controlled synthesis in continuous flow reactors, we demonstrated that high-aspect-ratio, flake-like ZnO provides superior antimicrobial activity and UV absorption compared to conventional spherical ZnO. Key process parameters, including reagent purity, reaction conditions, and flow rates, were meticulously preserved across scales to ensure product consistency.
Our results confirm that ZnO synthesized across different scales exhibits consistent morphology, crystallinity, and antimicrobial efficacy, as verified by SEM, XRD, disk diffusion tests, and ISO 22196 antimicrobial assessments. Additionally, our technoeconomic analysis highlights the efficiency of our approach, reducing scale-up investment costs by 86%, operating costs by 55%, and the time to breakeven by 94%, demonstrating the commercial viability of our process.
This work establishes a robust and scalable route for producing high-quality antimicrobial ZnO, enabling its application in coatings, medical devices, and advanced materials. The successful translation of our synthesis strategy from gram-scale R&D to ton-scale manufacturing underscores the potential of flow-reactor-based nanomaterial production as a cost-effective and scalable alternative to conventional batch synthesis.
References
- Espitia, P.J.P., Soares, N.F.F., Coimbra, J.S.R., …, Cruz, R.S., Medeiros, E.A.A. Food and Bioprocess Technology. (2012) 5(5), pp. 1447–1464
- Ong, C.B., Ng, L.Y., Mohammad, A.W. Renewable and Sustainable Energy Reviews. (2018) 81, pp. 536–551
- Shetti, N.P., Bukkitgar, S.D., Reddy, K.R., Reddy, C.V., Aminabhavi, T.M. Biosensors and Bioelectronics. (2019) 141, 111417
- Jiang, Z., Liu, B., Yu, L., …, Zhang, S., Li, W. Journal of Alloys and Compounds. (2023) 956, 170316
- Kadhim, M. J., & Abdulhussein, M. A. Materials Chemistry and Physics. (2020) 252, 123243
- Huang, L., & Wang, Y. Advanced Materials Interfaces. (2018) 5(12), 1800214.
- Jose, N.A., Kovalev, M., Bradford, E., … Chun Zeng, H., Lapkin, A.A. Chemical Engineering Journal. (2021) 426, 131345