by Nicholas A. Jose, Mikhail Kovalev and Alexei A. Lapkin
Abstract
The development of sustainable energy economies is blocked by the lack of stable electrical energy sources with high power and energy densities. Next-generation supercapacitors utilizing 2D layered double hydroxides (LDHs) promise to fill this need in hybrid and standalone architectures; however, despite their high power and energy densities, LDH supercapacitors have poor stability. New methods for creating robust LDH electrodes are necessary to prevent this degradation. Herein, the recently developed annular microreactor is used to synthesize defect-rich NiCoLDH nanocrystals. A simple, solvent-based method is used to rationally generate binder-free, superstructured thin films on Ni foam electrodes. Control over crystallite size, thinness, and orientation improves contact with the conductive substrate, increases reactivity, and improves structural stability. Optimized electrodes are fabricated with specific capacitances from 3000 to 5000 F g−1 at charging rates as high as 1000 A g−1, a performance that is retained after 20 000 cycles. This is twice as stable at 5000 times the current density of the most stable reported Ni-based supercapacitor. Ultimately, this study addresses key concerns in electrode development, introduces new approaches through reactor technology and solvent-mediated assembly, and opens new ground for more fundamental inquiries into the mechanisms of electron transport in 2D systems.
Introduction
The transition to a “net-zero” world requires the adoption of new and diverse energy storage methods.[1] However, conventional and emerging technologies, from lithium-ion batteries to fuel cells, are limited by their operating power ranges and sensitivities to fluctuating supply and demand.[2] Supercapacitors, electrical energy storage devices with power densities higher than current battery technologies (0.5–10 kW kg−1) and higher energy densities than conventional capacitors (1–10 kWh kg−1),[3] can fill this gap as standalone energy sources or in hybrid architectures for renewable energy storage infrastructures[4] and regenerative braking in automobiles.[5]
However, a primary challenge in the use of conventional supercapacitors, typically electric double-layer capacitors (EDLCs), is their low energy density.[6] To store a sufficient amount of energy, bulky cells with high weights and form factors must be used, limiting their use in mobile applications.[7] For example, activated carbon, a common electrode active material, possesses a specific capacitance of ≈10–300 F g−1.[8]
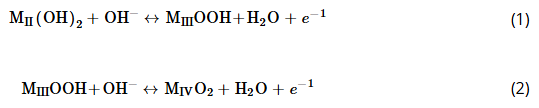
Unfortunately, state-of-the-art LDH supercapacitors suffer from a number of practical limitations. First, they possess low cycling stability. To compete with commercial carbon EDLC capacitors, capacitance must be retained for up to 100 000 charge/discharge cycles,[6] which no LDH-based supercapacitor reported in literature to date has achieved.[13] Many nickel-based LDH-based supercapacitor materials only report good stability up to 1000–5000 cycles, typically losing as much as 40% of their original capacitance.[14]
Capacitance reduction in LDH-based supercapacitors is attributed to two key factors: structural degradation caused by 1) the loss of intercalating ions and binding of LDH sheets and 2) the irreversible loss of lattice oxygen.[15] Charge build-up caused by poor electron transfer to the conductive substrates, which is impeded by the use of polymeric binders, also accelerates degradation. Finally, LDH often forms stacked, dense structures that have reduced available surface area and inhibit the transport of ions to and from active sites. In nickel-based LDHs, this is the transition from the water-intercalated “α” structure, to the nonintercalated “β” structure,[16] which are shown in Figure 1b,c.
Various methods have been used to increase surface area and stability, from the optimization of metal ion compositions,[15] the use of nanosized, anisotropic conductive supports like graphene and carbon nanotubes for increased surface area and conductivity,[17] the use of electrochemically or hydrothermally synthesis methods,[10] or the fabrication of unique, flower-like morphologies.[18] Wei et al. reported the highest known stability in a Ni-based LDH electrode, which was just 95% at a current density of 0.2 A g−1 over only 10 000 charge discharge cycles.[19]
Laboratory fabrication procedures are also often conducted with batch, hydrothermal reactors and multistep electrode coating protocols. Such processes are difficult to scale to industrial levels due to the higher costs of high-pressure and high-temperature batch processes, as well as their lower space–time efficiencies.
In this study, we investigate defect-rich or “amorphous” LDH nanoparticles and their superstructures, which have been reported to have higher stability and capacitance.[20] Defects, particularly edge sites, are known to be more active in electrochemistry,[21] catalysis[22] and adsorption.[23] Because they are less coordinated, edge hydroxyl groups (mono- and bicoordinated) are more labile and reactive compared to tri-coordinated hydroxyl groups at the basal surface, which are depicted in Figure 1d. Smaller crystallite sizes also may increase the stability of LDH structures by reducing the stress and strain developed during charging and discharging, thereby preventing structural degradation.[13] Recent innovations within our group in reactor technology have allowed us the capability to reliably and cost-effectively synthesize particles with high control over crystallinity, size, and shape.[24]
Furthermore, we examine the fabrication of superstructured thin films from LDH nanoparticles as way to improve stability, conductivity, and adhesion to substrates without the use of polymeric binders. In single-sheet thin films on metallic substrates, LDH would be less likely to form low-conductivity aggregates, may be tightly bound via electrostatic and Van der Waals forces, and could enable electron tunneling for improved conductivity.[25] Recent studies in our group[26] have shown how solvent alone can mediate the formation of complex and unique superstructures of 2D nanoparticles in dispersions, giving us a rational design method for fabricating such thin films.
Herein, we develop a new strategy for the synthesis of stable, high-rate capable LDH supercapacitor electrodes. Using a new continuous, room-temperature synthesis for NiCoLDH, and by coating electrodes without a polymeric binder, we reduce the process steps, energy, and resources needed for electrode fabrication. By controlling the shear rate of synthesis, dispersing solvent, and loading of the substrate, we can tune our particle and coating properties to produce capacitances close to theoretical maximums. We achieve stable performance even after 20 000 cycles at specific current densities as high as 1000 A g−1. This dramatic performance improvement is mechanistically explained by the increase in the number of reactive edge sites, disruption of LDH stacking, and low coating thinness.
Results and Discussion
NiCoLDH was synthesized via coprecipitation in the annular microreactor, in which metal salts are combined with NaOH at various flowrates. The purified NiCoLDH slurry was directly drop coated on nickel foam and dried in air in an oven. The dispersing solvent was varied between water, ethanol, and acetone. This method produced strikingly better capacitance and more stable performances, which were further optimized. This method uses fewer processing steps than conventional preparation techniques; such techniques commonly require LDH slurries to be dried multiple times and combined with several other reagents and solvents, such as N-methylpyrolidone, polyvinylidinefluoride, and acetylene black.[13, 27] This fabrication method only requires purification, solvent exchange, and one drying step. Furthermore, the presented synthesis takes place at ambient temperature (21 °C) in a continuous process. Compared to previous techniques for LDH synthesis, which require temperatures up to 90 °C, small batches, and hours of reaction time, our method is less time, space, and energy consuming.
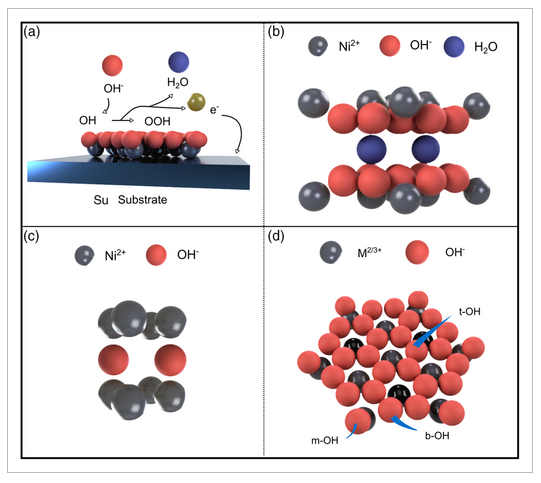
X-ray photoelectron spectroscopy (XPS) spectra seen in Figure 2a–c show peaks corresponding to Ni(OH)2, CoOOH. The Co 2p peaks were fit at 781 and 782.3 eV (Co 2p3/2), with satellites at 786 and 790.9 eV, as well as 797.0 eV (Co 2p1/2) with a satellite at 802.7, which can be attributed to both Co(OH)2 and CoOOH.[28, 29] The Ni 2p peak was fi at 856.2 eV (Ni 2p3/2) with a satellite at 861.9 eV, and 873.7 eV (Ni 2p1/2) with a satellite at 879.7 eV, matching reported values for Ni(OH)2[29] and CoOOH. The O 1s peaks were fit at 531.7, 532.8, and 529.72 eV, characteristic of Ni(OH)2, CoOOH, and Co(OH)2.[28, 29] The closeness of the characteristic peak positions of Co(OH)2, Co3O4, and CoOOH (Co 2p) makes it difficult to assign an exact oxidation state from XPS alone.
XRD patterns in Figure 2d show the characteristic reflections of α-NiCoLDH, which possess broad peak widths. The dispersing solvent had significant effects on the (001) peak position and broadness (FWHM). EtOH-NiCoLDH had the broadest and most left-shifted peak (θ = 9.7° center, FWHM = 6.54) compared to H2O-NiCoLDH (θ = 11.1, FWHM = 3.8) and acetone (θ = 11.3, FWHM = 5.0). While the shift of the (001) peak implies a larger basal spacing (≈1.37 nm), and the peak broadness suggests poor stacking of the LDH layers. Estimates of the crystallite size from the Scherrer equation and intercalation space are shown in Figure 2e.
The FTIR spectrum of the synthesized NiCoLDH shows peaks associated with α-Ni(OH)2, specifically the bending and stretching modes of intercalated water molecules, seen in Figure 2f.
The large peak widths are due to small lateral particle size and thinness. As shown in our previous study,[24] synthesis at high shear rates and high supersaturations results in uniform, monolayer LDH with particle sizes as low as 4 nm. These particles may self-assemble into larger crystals depending on hydrodynamic conditions during synthesis and postprocessing.
The increase in the (001) peak width and spacing with the introduction of organic solvents is due to the interruption of interlayer hydrogen bonding. Intercalating species are known to have a significant effect on LDH morphology and crystallinity.[30] Polar organic solvents like ethanol may replace water on LDH surfaces via hydrogen bonding to LDH surface hydroxyls with their organic groups oriented away at an angle,[31] which increases the spacing of LDH layers and reduces hydrogen bonding between layers. Alcohols are more effective in this process than solvents like acetone because they better align with each other, exposing nonpolar groups to surrounding water.[31]
H2O-NiCoLDH and Ac-NiCoLDH also have higher and more narrow (110) peaks, meaning that lateral size is also influenced by the dispersing solvent. Previous studies have shown that LDH can undergo lateral-oriented attachment, mediated by acid–base interactions and hydrogen bonding.[24] Ethanol also likely disrupts lateral-oriented attachment by aligning at particle edges, hindering hydrogen bonding and acid/base reactions.
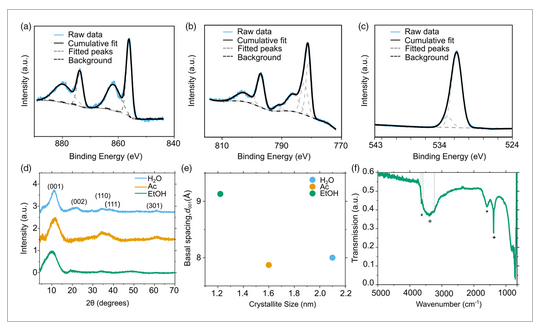
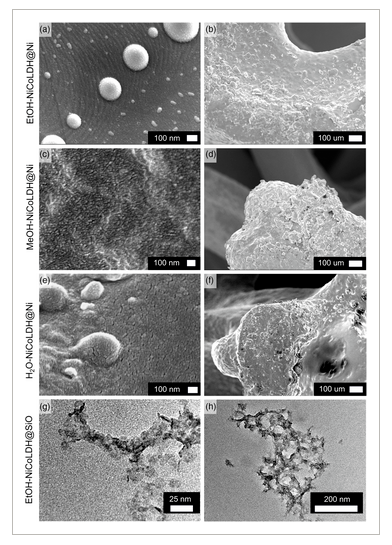
We began optimizing our LDH electrode by first varying the loading of the Ni foam electrode with EtOH-NiCoLDH. Electron microscopy was used to gather information on the structures of LDHs. Scanning electron microscopy (SEM) provides information on the microstructures where LDH particles form on the surface of Ni foam substrates. In contrast, transmission electron microscopy (TEM) analysis was used to collect information on the LDH particle dispersions themselves, in order to characterize the properties’ predrop casting. The combination of microstructure and nanostructure characterization helps rationalize observed changes in electrode performance, such as shifts in capacity, stability, and resistance. SEM images show that the LDH particles form a uniform coating on the Ni foam surface and may agglomerate into hemispherical structures of up to 500 nm in diameter, as seen in Figure 3a–b. TEM imaging shows that these particles are approximately 12.3 ± 3.1 nm in diameter and 3.4 ± 1.3 nm thick (Figure 3g,h and Figure S1, Supporting Information). In TEM imaging hemispherical aggregates were not observed, implying that the coating process on the Ni foam surface plays a role in their formation. Representative cyclic voltammetry curves as seen in Figure 4a show peaks at 0.19 and 0.27–0.36 V versus saturated calomel reference electrode (SCE), which correspond to oxidation of Co and Ni respectively.[32] Analysis following the Dunn model[33] of cyclic voltammetry curves shows that surface or capacitive charge storage increaseswith increasing voltage ramping rate, from 3% at 10 mV s−1 to 59% at 100 mV s−1 (as seen in Figure S2, Supporting Information) although at 100 mV s−1 ohmic losses and peak shift may cause some deviation from ideal values. This increase is consistent with pseudo/capacitive materials.[34] By conducting galvanostatic charge/discharge cycles at a current density of 100 A g−1, it was found that a loading of ≈0.05–0.10 mg cm−2 yielded optimal specific capacitances over multiple reaction conditions, as seen in Figure 4b. In this range, cycle times were significantly longer than the Ni foam substrate alone. The cyclic voltammetry (CV) voltage range was used to capture the properties of the electrodes over a wide voltage range. The galvanostatic charge discharge (GCD) voltage range was chosen from the optimal region within the voltage range. Above a voltage of 0.38 V, a relatively low amount of charge is stored.
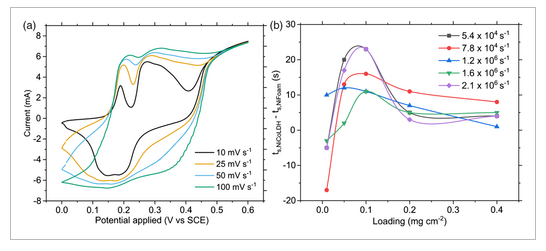
Optimization of loading is critical to ensure good contact with the conductive substrate. At high loadings, particles will form thick films with poor contact to the Ni surface. The effective loading is related to the available surface area of the substrate, which for Ni foam is approximately 0.5 m2 g−1.[35] For a 1 cm2 × 2 mm Ni foam substrate, this corresponds to an available surface area of ≈0.03 m2; the amount of 2D NiCoLDH with an approximate surface area of 1067 m2 g−1 to coat this area is 0.056 mg.[36] This approximates the optimal loading we determined, assuming the film is oriented with the basal plane on the Ni foam surface. Such a monolayer is highly effective due to the high exposed surface area to the electrolyte, low diffusion resistance, and close contact with conductive substrate, ultimately lowering the electron path distance and resistance.
It is important to discuss at this point the relevance of performance metrics gathered at mass loadings <1 mg. Inaccuracies in measurements of mass loading sometimes lead to the reporting of unreliable performance values;[37] in this study we have evaluated more than 100 samples over different conditions with three replicates each to show significance. Our method of determining mass loading by casting a known amount of slurry with a known concentration is also more reliable than directly weighing the electrode before and after casting on a microbalance, for which we found significant error. Our estimates of uncertainty from this method are approximately 5 μg cm−2; for current loadings of 100 A g−1, this leads to an uncertainty of 200–300 F g−1, depending on the mass loading and time measured, which correspond to the errors measured during experimentation.
It is also important to emphasize that the performance metrics gathered here are intrinsic to the material and not a functioning supercapacitor device, where an anode and casing are required. For commercial supercapacitors, energy/power densities include masses and volumes of other device components; for example, active carbon material accounts for only 30% of the total mass of a commercial packaged supercapacitor.[38] Energy and power densities are not reported in a Ragone plot to avoid this confusion.
Reactor shear rates were then varied from 9.1 × 104 to 2.8 × 105 s−1 by tuning the reactor flowrates. As seen in Figure 5a, capacitance varied nonmonotonically with shear rate, obtaining a maximum value at 8.4 × 105 s−1. This is likely because the particle size distribution, shape, and crystallinity of LDH particles vary as a function of shear rate. Because uniform and highly anisotropic nanoparticles are required for thin, highly ordered coating of substrates, optimization of shear rate is essential.
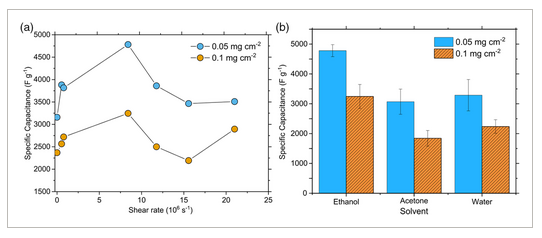
Shear rates where mixing is slower than the growth rate of LDH particles lead to polydisperse size distributions and lower-aspect-ratio particles. At the conditions used in this study, which are similar to those used in another study,[24] the characteristic growth time is ≈16 ms. Shear rates that accelerate oriented attachment of particles also lead to polydispersed size distributions and less anisotropic materials. At the optimal shear rate, the micromixing time (1.9–0.43 s) is lower than the characteristic growth time, leading to a more uniform size distribution and thus better coating of the Ni foam substrate. At higher shear rates, the aggregation and oriented attachment of LDH particles may be increased, leading to a less homogeneous particle size distribution.
We also evaluated the effects of different dispersing solvents on capacitance. Ethanol dispersions had significantly better performance than acetone and water dispersions, as seen in Figure 5b. As discussed previously, ethanol is most effective at hindering stacking and lateral self-assembly and increasing interlayer spacing; this leads to higher available surface area, more edge groups, and less diffusive resistance. SEM images shown in Figure 3c–f show that Ac–NiCoLDH and H2O–NiCoLDH form more disordered films on the surface of the nickel foam and lack the hemispherical aggregates that were observed with EtOH-NiCoLDH. This effect is interesting, as earlier in situ and computational studies on 2D metal organic frameworks showed that solvents play a key role in determining superstructure formation of 2D materials.[26] Further analysis in this area may be useful in explaining why different solvents impact material properties and electrode performance.
Using nanocrystalline LDH also increases reactivity of surface hydroxyl groups, due to the lower coordination of edge hydroxyl sites. The presence of edge sites and other defects may also significantly improve stability, by hindering crystallization of the β polymorph. In previous studies, aggregates of LDH nanoplatelets synthesized in mesoscale flow reactors were shown to possess superior thermal stability as well.[39] The number of edge sites increases exponentially with decreasing particle size as shown in Figure 6, where the calculated fraction of single and bicoordinated hydroxyl groups on a hexagonal 2D crystal is plotted against the lateral size of the particle. Although the higher reactivity of edge sites has been well characterized in chemisorption,[23] and electrochemical catalysis,[21] few studies have thoroughly explored their function in capacitors.
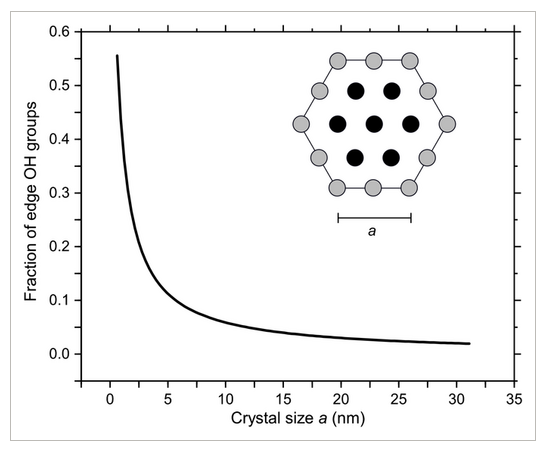
Edge hydroxyl group fraction as a function of crystal size, assuming hexagonal particles with side length a.
Using the optimal loading, reactor shear rate, and dispersing solvent, we then evaluated the cycling stability and capacitance over a range of current densities. Optimized EtOH-NiCoLDH@NiFoam possessed capacitances of 3,247 ± 401 and 4,781 ± 201 F g−1 at 100 A g−1, at loadings of 0.1 and 0.05 mg cm−2 respectively. These correspond to specific energy densities of 65.1 and 95.9 Wh kg−1 respectively. These values are close to the theoretical capacitance 4095 F g−1. The sample at 0.05 mg cm−2 may exceed the theoretical capacitance due to contributions from the Ni foam substrate or additional double-layer effects, which has been observed in previous studies with thin film Ni(OH)2.[10] Electrochemical impedance spectroscopy (see Nyquist plot in Figure 7c,d) shows low solution resistance (589 mΩ) and charge transfer resistance (44.8 mΩ), which were obtained from fitting (see Figure S3, Supporting Information).
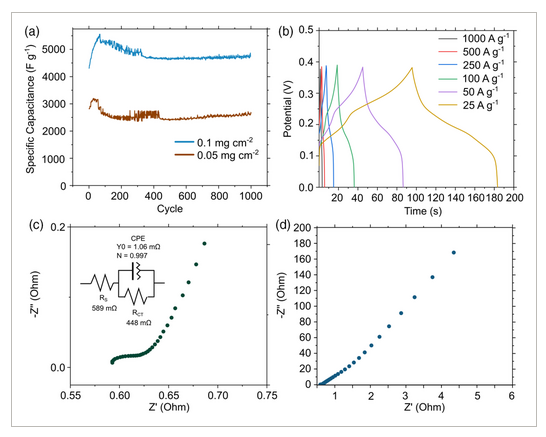
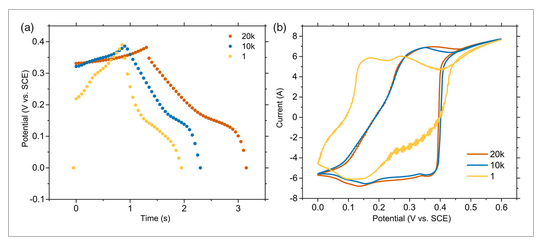
This low resistance is attributed to the low diffusion path to the thin-film surface and high reactivity arising from the abundance of edge hydroxyl groups. It should be noted that the resistance will change when the material is incorporated into an asymmetric supercapacitor device. After 1000 charge–discharge cycles at 100 A g−1, capacitances remained stable at 4745 (0.5 mg cm−2) and 2612 F g−1 (0.1 mg cm−2), seen in Figure 7a. The electrodes retained high specific capacitance at current loads as high as 1000 A g−1 with a capacitance of 4342 F g−1, seen in Figure 7b. As seen in Figure 7a, the specific capacitance initially increases and then decreases before stabilizing. Electrode materials are known to undergo a gradual process of activation, which consists of structural and electrochemical changes, which create electroactive sites as they are cycled through charged and discharged states. Electrodes are also known to degrade a certain extent during the course of cycling, resulting in the deactivation of reactive sites. Such changes can include reorientation of surfaces and changes in chemical functionalities. Zhang et al. previously showed that optimization of cycling can activate 2D MnO2 by restructuring the material into higher surface area morphologies.[40] In this case, we may observe an initial increase in capacitance as the electrode materials become activated due to this effect and then a decrease as they degrade. Verifying this effect in future in situ studies would help validate this. After 20 000 charge–discharge cycles at 1000 A g−1 (0.5 mg cm−2), capacitances seemingly increased to ≈3500 F g−1 as seen in Figure 8. A marked change was also observed in the cyclic voltammetry peaks, in which the nickel oxidation peaks increase and cobalt peaks decrease. This is possibly due to a restructuring of the electrode over long cycling times or possible leaching of the cobalt into the electrolyte. In the first CV cycle, a region of “noisy” behavior was identified upon discharge. This noise could have arisen due to structural changes during activation in the initial periods of cycling or from the release of residual air from the pores of the Ni foam electrodes.
Hemispherical aggregates on the surface of Ni foam may have competing effects on performance. While surface area may be increased by the hemispherical arrangement, the aggregates may have lower conductivity and be more prone to degradation. In future studies it would be worth developing a method to tailor the size of hemispherical aggregates and study the relationships between size, morphology, and performance.
The reduced capacitance and greater instability of the 0.1 mg cm−2 sample show that excess active material can lead to instability, likely by accelerating the transition to β-NiCo(OH)2, this and indicates that specific capacitance and stability are highly sensitive to loading and layer thickness. Being able to produce a monolayer film is crucial to enhancing performance and stability.
The large increases in electrochemical performance, particularly the low resistance and high stability, also suggest that more fundamental investigations may be required. Recent studies in 2D materials like graphene[41] and transition metal dichalcogenides[42] have indicated that the relative orientation of molecular sheets can dramatically influence electron transport and result in exotic electrical properties like superconductivity. Quantum tunneling has also been found to play a significant role in enhancing conductivity in 2D nanoparticle assemblies and is perhaps one factor behind the low resistance of the thin-film electrodes.[25] Future studies integrating this material into assembled small- and large-scale energy storage devices are also essential to validate device stability and efficiency. To use higher masses that are required for practical devices, a higher surface area, 3D-structured substrate could be used or an ultrathin foil.
Conclusion
To demonstrate the ability to tailor material properties and performance using the annular microreactor and solvent exchange, we synthesized 2D LDH electrodes for supercapacitor applications. By precipitating NiCoLDH at different shear rates, we also showed that capacitance also varies nonmonotonically with shear rate. At the optimal shear rate, a more uniform distribution of LDH particles leads to a more uniform and anisotropic coating of conductive substrates. Capacitance was also highly dependent on the dispersing solvent, which plays a key role in hindering the stacking and aggregation of particles during coating, enabling the formation of an ultrathin, possibly monolayer coating. In contrast to conventional electrode preparation methods reported in literature, we use a continuous, RT process without a binder, increasing the scalability of our method.
Further application work in the development of stable Ni-based electrodes should include the development of full cell configurations that enable high loadings of electrode material and more realistic measurements of stability. This could be accomplished by either the use of high-surface area-structured, 3D electrode substrates or with coiled, ultrathin substrates. This may be implemented commercially to reduce the cost and improve the performance of LDH-based electrodes in energy storage devices. In situ studies could also be conducted to analyze structural changes of LDH suspensions drying in different solvents, as well as how the electrodes change over the course of charge/discharge cycles. Fundamental physical studies may also shed light on how the changes in relative orientation, thinness, and crystallite size of the nanoparticle films affect the mechanisms of electron transport, explaining the significant increases in performance we observed in this study. A comprehensive, fundamental analysis of why these crystallite parameters, and thus the shear rate, affect NiCoLDH capacitance is also necessary for advancement in the field. Such a study would involve the use of in situ analytics for measurements of particle crystallinity and morphology during charge/discharge cycles, as well as techniques for accurately characterizing nanocrystalline defects and oxidation states, such as in situ XPS.
Experimental Methods
LDH Synthesis
Reagent grade nickel nitrate(II) hexahydrate, colbalt(II) nitrate hexahydrate, and sodium hydroxide were purchased from HCS Scientific. A metal ion solution (solution A) of Ni2+ and Co2+ and a basic solution (solution B) of NaOH and Na2CO3 were prepared in deionized water at approximately 21 °C. The molar ratio of Ni2+: Co3+ was fixed at 1:1, with a total concentration of metal ions of 0.1 m in solution A. The concentration of solution B was 0.21 m, which yielded a final product pH of 10.8.
Synthesis was conducted in the annular reactor previously described in other studies.[24, 43] Three quartz tubes were oriented coaxially to create two annular flow zones, coupled with T-fittings. Liquids were delivered to the reactor using a KDS Legato Dual Syringe Pump using disposable plastic 10 mL Terumo syringes. Compressed dried air was filtered through a 200 μm filter (Swagelok) and delivered to the reactor using a Sierra SmartTrak C50L Mass Flow controller (20 L min−1 max, 2% accuracy).
Solutions A and B were pumped through the first and second t-fittings of the reactor at equal flow rates. The total liquid flow rate QL was varied from 8 to 20 mL min−1 and the gas flowrate QG from 0.5 to 1.75 L min−1. The collected suspension was centrifuged immediately after synthesis in a Hanil 514 R centrifuge at 6000 RPM for 3 min. The collected solids were rinsed three times with deionized water to purge unreacted ions.
Electrode Fabrication
To prepare binder-free NiCo@NiFoam electrodes, the purified solids from synthesis were washed and resuspended in different solvents—deionized water (<18 μΩ, Millipore), ethanol (95%, Merck) and acetone (99.8%, Merck). The concentrations of the suspensions were then determined gravimetrically. Aliquots were then drop cast onto 1 cm × 1 cm × 0.75 mm Ni foam substrates and dried for 2 h (acetone, chloroform, and ethanol suspensions at 60 °C and water suspensions at 110 °C in air). Loadings were varied from 0.01 to 0.40 mg cm−2. All electrodes were prepared on the same day of synthesis, approximately 4 h after synthesis of LDH.
Electrochemical Characterization
Electrochemical measurements were conducted using a three electrode cell using a 1.5 × 1.5 cm2 Pt foil as the counter electrode, a SCE, and the coated nickel foam as the working electrode in 6 m KOH solution. All experiments were conducted on an Autolab electrochemical workstation running on Nova 2.0 software. CV was conducted from 0 to 0.6 V versus SCE under scan rate of 25–100 mV s−1 for a period of five cycles. GCD tests were conducted from 0 to 0.38 V versus SCE initially for a minimum of two cycles and maximum of 20 000 cycles. Electrochemical impedance spectroscopy tests were conducted from 100 000 to 0.1 Hz at 0.325 V versus SCE with an oscillation amplitude of 0.01 V. The EIS spectra was fit to a simplified Randles cell model using Nova 2.0’s regression functionality.[44]
Transmission Electron Microscopy
Suspensions were ultrasonicated in an Elma Ultrasonic S100h bath for 1 min to disperse large agglomerates, dropped onto silicon monoxide films purchased from Agar Scientific, and dried at room temperature. High-resolution images were taken with a JEOL 2100 F FETEM at 200 kV. Samples were imaged for brief time periods under low current density to prevent radiation-induced restructuring. Silicon monoxide films were used as substrates because it provides better dispersion of ultrathin LDH particles, which tend to agglomerate and recrystallize upon drying on carbon substrates.[39] The drawback of silicon monoxide substrates, however, is their increased thickness and density and thus lower contrast of the images. Particle size distributions of the diameter and thickness of the platelets were evaluated using ImageJ, by measuring lateral and longitudinal dimensions.
Scanning Electron Microscopy
Suspensions were diluted in their respective solvent and drop cast on silicon substrates, dried at room temperature, and coated with Pd in a Cressington sputter coater for 30 s. Images were taken with a JEOL JSM-5600LV FESEM at 5 kV.
X-Ray Diffraction
Suspensions were deposited onto a nonreflective silicon wafer (100) and dried at 80 °C in air. The powder X-Ray diffraction pattern was collected with a Brucker D8 Advance Powder Diffractometer using Cu Kα radiation (λ = 1.5418 Å) at 40 kV from a 2θ of 3° to 70° with a scanning resolution of 0.02° s−1. Basal spacing was estimated from the (001) reflection using Bragg’s law,[45] which is a standard technique for characterizing LDH stacking.[46]
X-Ray Photospectroscopy
XPS was conducted using a Kratos AXIS Ultra electron spectrometer from Kratos Analytical Ltd. using 75 W Al Kα radiation at a 90° angle. The survey scan was completed at 1100–-5 eV with a step size of 1 eV. Elemental scans were completed at a step size of 0.1 eV. Sample was prepared by drop casting an ethanol suspension onto a glass slide and drying at ambient conditions.
Acknowledgements
The authors would like to acknowledge Professor Hua Chun Zeng of the National University of Singapore for providing access to characterization facilities and advisement over the course of the project. This project was funded by the National Research Foundation (NRF), Prime Minister’s Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) program as a part of the Cambridge Centre for Advanced Research and Education in Singapore Ltd (CARES).
Conflict of Interest
A patent for the reactor used in this study is pending (WO2019158932A8) and is being commercialized by N.A.J., M.K., and A.A.L.
References
Follow the link to the article below to click through to the references via the online article